Self-Sampling Devices
V-Veil UP2TM Oral Veil Device
- Collection of oropharyngeal secretions through deep expectoration
- HPV, STI and Tuberculosis detection
- Veil efficiency: +20% of DNA release
- Compatible with UP2 RetrofitterTM tube

V-Veil UP2TM Anal Device
- For women and men, including MSM
- Veil efficiency: +20% of DNA release
- 96% satisfaction with Anal Veil and 94% usability
- Anatomic curve design and creasing Veil shape for optimal collection
- Available in 2 sizes (long and short) and with cap or with pierceable aluminum foil

V-Veil UP2TM PENILE Device
- Collect safely from urethra and the skin of the penis for detection of HPV and STI
- Veil efficiency: +20% of DNA release
- Technology available for self-sampling and clinician
collection - Compatible with UP2 RetrofitterTM tube
- Available with cap or with pierceable aluminum foil

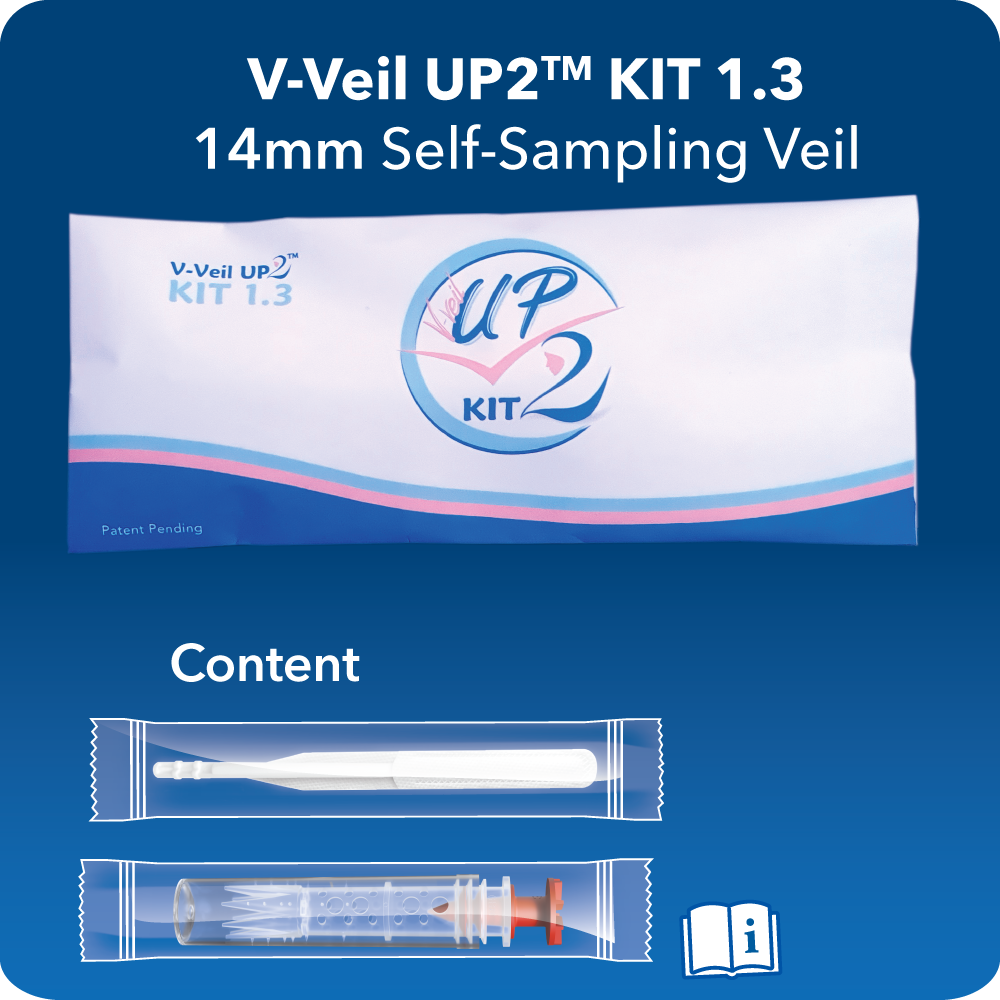
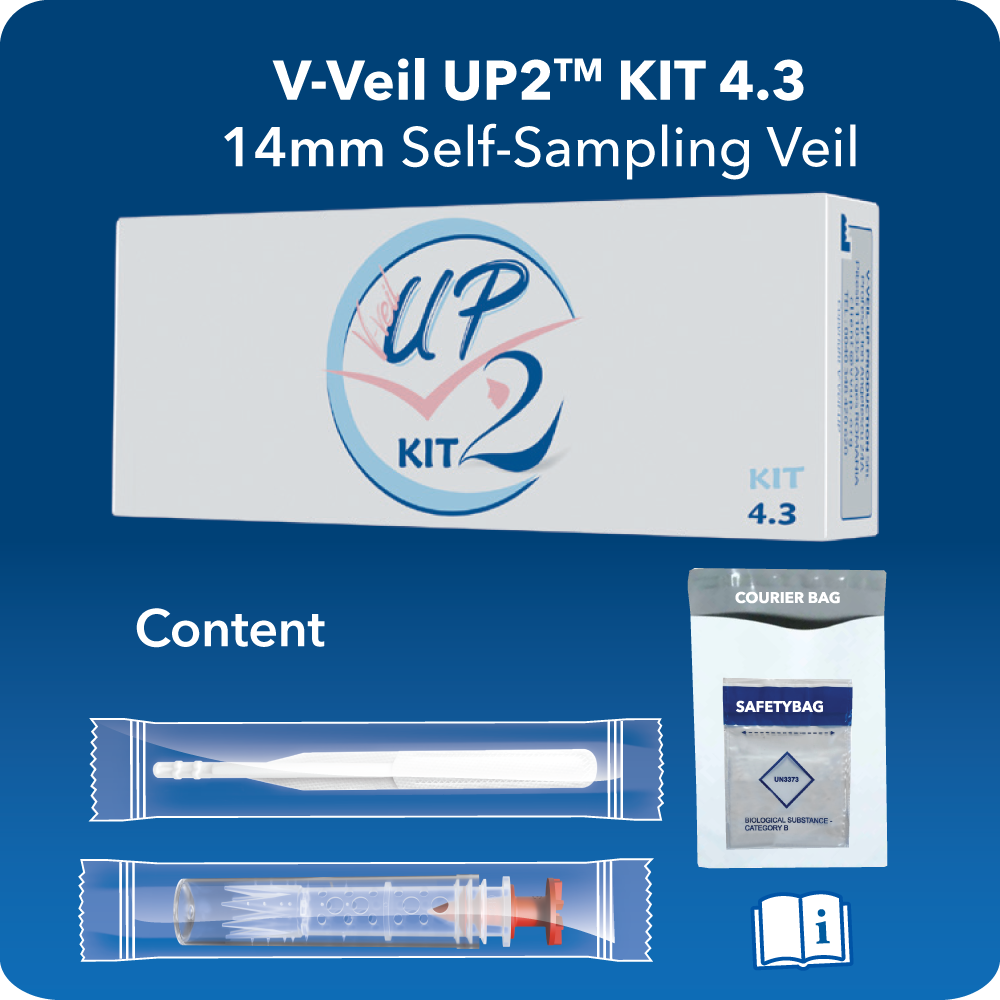
V-Veil UP2TM KITs
Are self-sampling procedure packs used to collect secretions, various fluids and substances from the vaginal cavity and cervix, for diagnosis by laboratory tests.
- Self-Sampling Medical Devices CLASS I according to Regulation (EU) 2017/745 (CE mark under the responsibility of the manufacturer).
- Produced in EN ISO 13485:2016 certified facility, in ISO 8 clean rooms.
- V-Veil UP2TM is listed as medical device for self-sampling into technology landscape report of Unitaid: Screening precancerous lesions for secondary prevention of cervical cancer.
- The Patented Veil Technology is engineered for higher yield: 0.7% DNA and 81.3% protein release
- The stability of the V-Veil UP2TM collected sample in dry media without any kind of liquid) at temperatures between 4 to 40°C is 30 days and at 50°C is 7 days.
- V-Veil UP2TM is simple, safe, and respects the privacy of all women.
- For women as final user, the KIT 4.1 and KIT 4.3 are available for on-line buying, in EU, at test-hpv.shop